BACKGROUND AND SIGNIFICANCE
Myelodysplastic syndrome (MDS) is a heterogeneous group of blood cancers of myeloid progenitor cells. They vary widely in genetic landscape, risk of progression to acute myeloid leukemia, and risk of death, as predicted by the revised international prognostic score (IPSS-R). The hypomethylating agents (HMAs) azacitidine (AZA) and decitabine (DEC) are the standard of care for treatment of high risk (HR)-MDS, however complete response rates are <15% and most patients (pts) eventually progress. Outcomes after HMA failure are dismal.
Defective apoptosis is a major feature of MDS. Increased expression of anti-apoptotic members of the BCL-2 family (BCL-2, BCL-X L and BCL-W) correlates with disease progression and poor outcomes. The addition of venetoclax (VEN), a selective BCL-2 inhibitor, has shown benefit when added to AZA in pts with relapsed or refractory (R/R) MDS (Zeidan, et al., AJH, 2023). However, patterns of BCL-2 family proteins are heterogeneous and response to inhibition of BCL-2 family proteins depends on the balance of pro- and anti-apoptotic proteins.
Navitoclax (NAV) is a broad oral inhibitor of BCL-2, BCL-X L, and BCL-W. Increased expression of BCL-X L has been shown to make MDS cells insensitive to VEN. Further, VEN-resistant myeloid cell lines derived by prolonged VEN exposure showed up-regulation of BCL-X L, driving acquired drug resistance in these cell lines. Thus, the addition of NAV to the combination of VEN and HMA is a logical strategy to induce response in pts with R/R after HMA with or without prior VEN exposure.
STUDY DESIGN AND METHODS
We will perform a phase Ib/II, dose escalation and expansion study to evaluate escalating doses of NAV in combination with standard dose VEN and DEC in pts with R/R HR-MDS after failure of HMA (NCT05564650). The primary objective of the phase Ib portion of the study will be to evaluate the safety profile and determine the recommended phase II dose (RP2D) of NAV in the combination. The primary objective of the phase II portion of the study will be to evaluate the efficacy of combination therapy NAV/VEN/DEC in pts with R/R HR-MDS after failure of HMA/VEN.
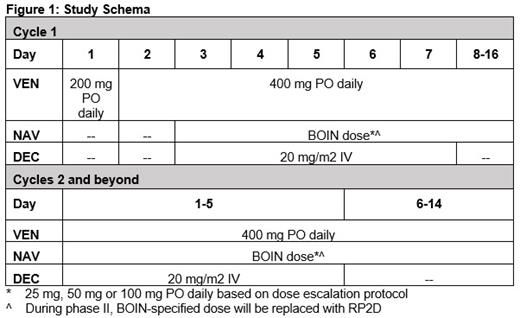
During cycle 1, to limit the risk of tumor lysis, pts will be treated with a short dose ramp-up with VEN 200 mg daily on day 1 and 400 mg daily on days 2-16, DEC 20 mg/m2 on days 3-7 and NAV 25 mg, 50 mg or 100 mg daily on days 3-16 during cycle 1. During cycles 2 and beyond, dosing will be similar to cycle 1 without dose ramp-up ( Figure 1). We will employ the Bayesian optimal interval (BOIN) design to identify the maximum tolerated dose and RP2D of NAV. Subsequently, the study will proceed to phase II to further evaluate the safety and efficacy of the combination.
The study will enroll pts with IPSS-R ≥3 who have been previously treated with HMA/VEN. Pts in the phase I portion of the trial may be enrolled if they have not received VEN. Pts must have adequate performance status and organ function. Pts with a WBC >25,000 cells/µL and those taking prohibited medications will be excluded. Correlative studies will be performed to determine the impact of treatment on molecular targets and will include profiling of BCL-2 family members and single cell gene expression patterns pre- and post-treatment and at relapse. Pts who achieve a complete response (CR) or modified CR (mCR) will continue on the study until disease progression, dose limiting toxicity, availability of an alternative therapy or withdrawal of consent. Pts who have hematologic improvement but who do not attain CR or mCR can continue the study at the discretion of their treating physician in conjunction with the primary investigator.
OffLabel Disclosure:
Keiffer:Cyteir Therapeutics: Research Funding; Prelude Therapeutics: Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding. Meyer:CellCentric: Research Funding. Eischen:Abbvie, Inc: Research Funding. Binder:Janssen: Research Funding, Speakers Bureau; Karyopharm: Speakers Bureau. Porcu:BioGene: Membership on an entity's Board of Directors or advisory committees; Kymera: Membership on an entity's Board of Directors or advisory committees; Kyowa: Consultancy; Dren-Bio, ADCT, Lilly-Loxo, Viracta, Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Ono: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma: Consultancy; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma, Ono: Honoraria; Teva: Research Funding; Innate Pharma: Research Funding. Wilde:Gilead: Research Funding. Kasner:Astellas: Membership on an entity's Board of Directors or advisory committees; Kartos/Telios: Research Funding; Gilead: Research Funding; Pfizer: Research Funding; Kronos: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding. Palmisiano:Genentech: Research Funding; Abbvie: Consultancy, Research Funding; Rigel: Consultancy.
The combination of hypomethylating agent and venetoclax is off-label for the treatment of myelodysplastic syndrome.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal